Small-Scale Fisheries and Food Security: Preventing Overexploitation
By James Keegan, RJD Intern
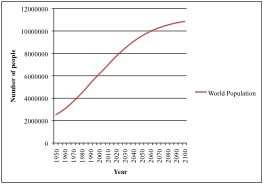
Artisanal, or small-scale, fisheries provide food and employment to hundreds of millions of people in developing countries, making these fisheries’ sustainability essential (Johnson et al. 2013). In tropical developing countries, 60% of the people depend on fish for 40% or more of their protein demand (Hussain et al. 2010). Growth in fisheries in the developing world outpaces growth in agriculture (Garcia et al. 2010). With a growing world population, persistent problems of hunger and malnutrition afflicting developing nations will only increase, so food security is a major societal and international concern (Garcia et al. 2010). Fishery resources are important sources of nutrition for many low-income populations in rural areas, and with such a large number of people relying on small-scale fisheries to alleviate poverty and contribute to food security, their international importance continues to increase.
Effective consumption in developing countries may be even higher than current statistics show because frequently small-scale fisheries do not report their catch (FAO, 2014). This is often the result of subsistence fishing, in which fishermen directly rely on their catch for food. Moreover, many fishermen practice on a part-time or occasional basis, and so a census may not record them as fishers, ultimately underestimating their number (FAO, 2014). With a more accurate estimation of small-scale fisheries’ catch, their already substantial global importance would be magnified.
Because they encompass a large number of people living in communities with low adaptability and heavy dependence on fish for food, small-scale fisheries are the most vulnerable type of fishery (FAO, 2014). Small-scale fisheries face many problems such as habitat degradation, climate change, lack of financial support, poor gear and infrastructure, and the lack of access to markets (Daw et al. 2012), (Halafo et al. 2004). Moreover, many fisheries suffer from an excess of fishing effort, endangering their long-term sustainability. It is particularly difficult to address excessive fishing effort in small-scale fisheries, where there are limited options for controlling access to fish. (Daw et al. 2012).
Usually, overexploitation of fish stocks result from unsustainable practices. This includes illegal, unreported, and unregulated fishing (IUU), which threatens both the local ecosystem and the socio-economic condition of the small-scale fishing communities (FAO, 2014). IUU reduces the quality and quantity of available catch for legitimate fishers, and often contributes to poverty and food insecurity (FAO, 2014). Another issue affecting sustainability of fisheries is the post-harvest loss of fish, which can occur at any stage of the supply chain. Recent investigations have revealed a direct relationship between high fish losses and increased fishing effort which fisheries use to compensate for these losses (FAO, 2014). Moreover, because of their inefficient structure, small-scale fisheries incur greater losses than the large-scale fisheries (FAO, 2014).
Proper management can alleviate these unsustainable practices that lead to overexploitation. The management of small-scale fisheries, with their fundamental components of population, poverty and food security, remains particularly problematic, and many small-scale fisheries remain practically unmanaged (Garcia et al. 2010). However, management of small-scale fisheries has improved, and recent approaches have shown success. Institutions that manage small-scale fisheries can be locally based, state controlled, or a mix of the two. The latter arrangement, known as co-management, is the preferred approach for sustainability (Kosamu, 2015). Bottom-up implementation of new management arrangements between the state and the local community may make it easier for the two to agree on an objective, as well as help the fishers adapt to the new system (Aburto et al. 2013). Although government management is helpful, a case analysis showed that the sustainability of small-scale fisheries depended on the strength of the collective capital of the local communities (Kosamu, 2015). With weak local capital, government involvement made no difference, and the fisheries were unsustainable in all cases (Kosamu, 2015). This indicates that governments can help small-scale fisheries effectively by protecting and supporting local institutions.
In addition to effective management, the availability of alternative food and income sources to local communities can reduce fishing pressure to a sustainable level (Kronen et al. 2010). National economic development and communal income sources affect local resource exploitation, and thus, the overexploitation of fish stocks. Economically poorer regions with fewer alternative employment opportunities will suffer more from overfishing. In support of this, recent studies have found higher fish biomass densities near sites with greater infrastructure, owing to an economy less reliant on natural resource extraction (Daw et al. 2012).
Small-scale fishery sustainability remains essential to developing nations, particularly in response to growing global populations. Implementation of proper management practices as well as support of local economies will ease the pressure on local fish stocks. With sustainable fisheries, food security will increase, and the quality of life for the local community will follow.
References:
Johnson, A. E., Cinner, J. E., Hardt, M. J., Jacquet, J., Mcclanahan, T. R., and Sanchirico,J. N. (2013). Trends, current understanding and future research priorities for artisanal coral reef fisheries research. Fish Fish. 14, 281–292. doi:10.1111/j.1467-2979.2012.00468.x
Hussain SA, Badola R (2010) Valuing mangrove benefits: Contribution of mangrove forests to local livelihoods in Bhitarkanika Conservation Area, East Coast of India. Wetlands Ecology and Management 18: 321–331. doi: 10.1007/s11273-009-9173-3
Garcia, S. M., and Rosenberg, A. A. (2010). Food security and marine capture fisheries: characteristics, trends, drivers and future perspectives. Philos. Trans. R. Soc. B 365, 2869–2880. doi: 10.1098/rstb.2010.0171 FAO (2014)The State of World Fisheries and Aquaculture 2014. Rome 223 pp.
Daw, T.M., Cinner, J.E., McClanahan, T.R., Brown, K., Stead, S.M., Graham, N.A.J., Maina, J. (2012). To Fish or Not to Fish: Factors at Multiple Scales Affecting Artisanal Fishers’Readiness to Exit a Declining Fishery. PLoS ONE 7: e31460. doi:31410.31371/journal.pone.0031460.
Halafo, J.S., Hecky, R.E., Taylor, W.D., 2004. The artisanal fishery of Metangula, Lake Malawi/Niassa, East Africa. African Journal of Aquatic Science 29, 83-90
Kosamu, Ishmael B.M. (2015) Conditions for sustainability of small-scale fisheries in developing countries. Fisheries Research 161, 365-373.
Aburto, J., Gallardo, G., Stotz, W., Cerda, C., Mondaca-Schachermayer, C., Vera, K. (2013) Territorial user rights for artisanal fisheries in Chile – intended and unintended outcomes. Ocean & Coastal Management 71, 284-295.
Kronen, M., Vunisea, A., Magron, F., McArdle, B., 2010. Socio-economic drivers and indicator for artisanal coastal fisheries in Pacific island countries and territories and their use for fisheries management strategies. Marine Policy 34, 1135-1143