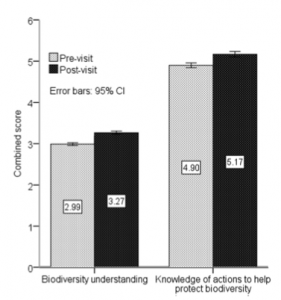
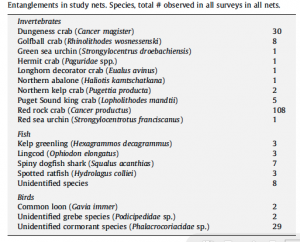
By Kyra Hartog, RJD Intern
1. Introduction
Marine algal species produce a variety of compounds that are ultimately beneficial to human health. These compounds are often produced as secondary metabolites [1], meaning they are not essential to the algal species’ survival but benefit the organism in some way. These compounds include, but are not limited to, polyunsaturated fatty acids and carotenoids, as well as compounds with antibiotic and antifungal activity. Those compounds with antibiotic and antifungal activity are being investigated for use as components in anti-fouling paints for maritime industries around the world [1]. Polyunsaturated fatty acids are being studied in relation to their benefits to human health including their potential anticancer activity [2] and their potential for treatment of the symptoms of cystic fibrosis [3]. Carotenoids also have great potential for benefits to human health including treatment of degenerative diseases like macular degeneration and the development of cataracts [4, 5]. Both polyunsaturated fatty acids and carotenoids can be found in algal species, which may provide a less expensive, more efficient mode of production for these compounds [6, 7]. Various algal species are also being studied as bases for biofuels that are more sustainable than current terrestrial options including oil palms, corn, and sugar cane [8].
Though marine products appear to have limited historical use as herbal remedies and medicinal products, a few instances have been reported in the case of marine algal species. Algal metabolites have been studied and developed further as technology to extract and bioassay these metabolites has developed. Marine algae are defined as eukaryotic macroalgae and microalgae for the purpose of this review. Prokaryotic “blue-green algae” (Cyanobacteria) are beyond the scope of this review.
2. Historical use of algae as herbal remedies and supplements
Of the many plant-based herbal remedies used throughout history, only a few have been derived from algal species. Inuit tribes in Nunavut, Canada used parts of a brown algae Laminaria solidungula as a general health supplement [9]. Members of the Rhodophyta division, Chondrus crispus and Mastocarpus stellatus, were used in Irish folk medicine as part of a beverage popular for treating colds, sore throats, and chest infections, including Tuberculosis [10]. They were also boiled in milk or water as remedies for burns and kidney issues [10]. Juice from another red algae, Porphyra umbilicalis, was used as a cancer remedy, particularly breast cancer. It was also used in the Aran Islands as a remedy for indigestion in people and constipation relief in cows [10].
3. Polyunsaturated Fatty Acids (PUFAs)
One typically thinks of marine polyunsaturated fatty acids (ω-3 and ω-6) as coming from oily fish like salmon and anchovies. Marine microalgae also represent a great source of these long chain PUFAs including docosahexaenoic acid (DHA) and eicosapentanoic acid (EPA) which play several important roles in the human body. DHA has been linked to brain development support in infants and may offer other protective functions to the brain later in life [11]. EPA gives rise to anti-inflammatory eicosanoids, which play crucial roles in the immune system, cardiovascular function, and cell communication in general [12].
3.1. PUFAs and Cancer
Marine derived PUFAs have three potential avenues for use in relation to cancer treatment: as an adjuvant for chemotherapy treatment, as compounds with direct anti-cancer effects, or as supplements to ameliorate the secondary effects of radiation and chemotherapy treatments [2]. The direct anti-cancer effects are specifically against tumors through inhibition of angiogenesis and metastasis [13]. These PUFAs were originally thought to have anti-cancer activity due to the low incidences of cancer reported in areas like Japan and the Mediterranean, where n-3 and n-6 levels are high in the diet [14]. The anti-inflammatory nature of the eicosanoids form from the metabolism of EPA is likely the source of the anti-cancer effects seen with these PUFAs. The eicosanoids reduce damage caused by oxidative stress and inhibit the COX-2 inflammatory pathway [2]. EPA and DHA have also been shown to protect tissues that are not the targets of chemotherapy treatment and increase tumor sensitivity to certain cancer treatments [13]. EPA was also shown to increase muscle mass in patients with wasting syndrome, or cachexia, associated with chemotherapy [15]. Though the correlation between increased muscle and body mass and PUFA supplementation may not have been significant in each and every study conducted, overall quality of life was certainly improved in all patients who received supplements [2].
3.2. Algal DHA and Treatment of Cystic Fibrosis Symptoms
Cystic fibrosis is a genetic disease in which mucous membranes, namely in the lung and intestines, do not function properly, causing mucous build-up. Patients with this disease have been found to have lower than normal levels of DHA and arachidonic acid (ARA) in their mucous membrane tissues as well as the blood [3]. This may be due to incomplete digestion of PUFAs as algal DHA supplements appear to be efficiently absorbed by patients with the disease [16]. Lung disease associated with CF is very inflammatory (high levels of ARA) so an increase in DHA derived anti-inflammatory compounds may lead to improvements in lung function by decreasing the ratio between ARA and DHA [3]. Algal DHA is beneficial to CF patients because it manifests fewer gastrointestinal side effects and is more compliant than similar doses of fish oil derived DHA. The doses of algal DHA can also be delivered without increasing pancreatic enzymes doses as well [3]. Algal DHA was found to deliver to red blood cells and plasma at an equal level to fish oils from cooked salmon [11].
3.3. Production of Algal PUFAs
Marine microalgae are a very good source of various PUFAs including EPA, DHA, ARA, and γ-linolenic acid. Certain species and algal strains can be selected for the type and quantities of the PUFAs they produce by manipulating the conditions in which the algae are cultured [6]. Ward and Singh [18] outlined the various species and the compounds they produce in their review of alternate sources of omega 3/6 oils: microalgae of the genera Phaeodatylum and Monodus are good sources of EPA; Schizotrychium species are stable sources of DHA for use in aquaculture, poultry and livestock feeds [19]. One of the problems with algal EPA production is that those species that accumulate EPA in the most available form, triglycerides, are obligate phototrophs, which require expensive photobioreactors for growth [19]. This may be remedied by genetic engineering technology that allows phototrophic species to be converted to heterotrophic species that require much less expensive fermenters for growth and are not hampered by the need for sunlight [18]. Heterotrophic cells can also grow in much higher cell densities compared to phototrophic bacteria because they don’t need sunlight for growth [18].

A photbioreactor set-up for the cultivation of microalgae
Image source: Wikimedia Commons
4. Antibiotic and Antifouling Activity
A study of extracts from Puerto Rican seaweed species showed 64% of the compounds assayed had some level of antibiotic activity [20]. These levels ranged from activity against a single species to activity against the entire range of bacterial species tested. This activity can be contributed to a variety of compounds with the most highly active being brominated compounds in Asparagopsis taxiformis solutions. Though the majority of species tested for antibiotic activity exhibited inhibition against only 1 or 2 microorganisms, 61% of the algae were active against the Gram-positive bacteria Bacilus subtiles and Staphylococcus aureus. Antibiotic activity was evenly distributed against the species in the divisions Rhodophyta, Chlorophyta, and Phaeophyta [20].
Secondary metabolites from marine algae also have activity against bacteria, other algae, fungi, protozoans, and macro species like barnacle larvae. These activities may contribute to algal metabolites making a good source for anti-fouling compounds as all the groups listed above participate in the formation of biofilms on maritime industry properties [1]. Two compounds from the red algae Laurencia rigida, elatol and deschlorelatol, were found to have strong activity against settlement of invertebrate larvae like that of barnacles and oysters [21]. A lactone compound from the brown algae Lobophora variegata, known as lobophorolide, has strong promise as an anti-fungal agent that is environmentally friendly for use in anti-fouling paints [21]. Compounds must meet the standards of the EC Biocide Directive for safety of registered toxins if they are to be used in commercial anti-fouling paints. Isolation of these compounds is very expensive but the solution may lie in genetic engineering. It allows for a safe supply and the potential for development of new compounds to remedy the ever-present problem of biofouling in the maritime industry [1].
5. Carotenoids
Carotenoids are pigment compounds that generally give a yellow, orange, or red color. They are synthesized by plants and algae and may play a role in photosynthesis. These compounds act as antioxidants, reducing stress from oxidative damage. Their bioactivity lies in their physiochemical properties, which depend on the structure of the molecule. Carotenoids also contribute to algae’s nutritive value in feed for aquaculture and animal farming. These values have made algae a potential nutraceutical for human use [7]. Some microalgae of the division Chlorphyta accumulate carotenoids as part of their biomass, including Dunaliella and Haematococcus species [22]. Dunaliella salina is a particularly good natural source of β-carotene, which has been shown to reduce the risk of cancer and degenerative diseases in humans [4, 23]. D. salina is currently being grown for production in open ponds [4, 6]. Haematococcus pluvialisis is one of the richest natural sources of astaxanthin and can be cultivated at a large scale for production of the compound [23]. Lutein is one of the most important carotenoids in foods and for humans. It is used as an additive in aquaculture and poultry operations and may be effective against a variety of disease including cataracts, macular degeneration, and early stages of atherosclerosis [5, 7]. Strains of the green microalga Muriellopsis are the most promising source for algal lutein accumulation and production systems are being developed [7].
Good candidates for algal production of carotenoids will have the same properties as those for production of PUFAs: high cell densities, efficient growth with minimal light, high percentage of desired compounds per cell. Genetic engineering is also an option for carotenoid production but no significant improvements in manipulation of eukaryotic microalgae has been seen so far. Further research and growth studies are required to realize marine algae’s potential for large-scale production [7].

[D. Salina.jpg] Natural salt ponds containing Dunaliella salina. The red color is due to their high levels of β-carotene.
6. Biofuels
Most biofuels on the market today are derived from terrestrial sources like oil palms and corn. These biofuels are disadvantageous in that they put a strain on food markets, contribute to water shortages by taking water away from other operations, and further the already rampant destruction of rainforests for resources. Microalgae offer a more economical and environmentally friendly option for the production of biofuels. They have several characteristics that make them more viable biofuel source compared to terrestrial options: they can produce oils year round, they grow in aqueous media and need less water, they can be cultivated on otherwise agriculturally unusable land, and they have a high oil content based on dry mass (20-50%). They may also be able to remove carbon dioxide from the atmosphere and their nitrogen waste may be used as fertilizer [8]. Oil yields may be increased through manipulation of algal growth conditions including temperature, pH, light, carbon dioxide levels, and harvesting methods [6]. Different strains will have the highest oil yield, the highest carbon dioxide fixation rate, the most efficient growth cycle, etc. so strains must be selected for a balance of these traits to create the best overall strains for biofuel production [8]. Once the various strains are produced and a biomass is obtained, the mass must be converted, either thermochemically or biochemically into the usable products for biofuels. There are a variety of conversion methods depending on the starting product and the desired end product [24]. Microalgal production methods are still relatively expensive so further research and engineering are needed in order to choose strains that will be the most effective for biofuel production. The environmentally friendly nature of algae-based fuels is perhaps the most attractive aspect of their use as current options such as palm oil require clear-cutting of rainforests, killing thousands of endangered animals.
7. Conclusions
Microalgae are a vast, largely untapped resource for a variety of natural products. These products may be used for everything from human health supplements to animal feeds to biofuels. Some of the most valuable compounds derived from marine algae are the polyunsaturated fatty acids, carotenoids, antibiotic compounds, antifungal compounds, antifouling compounds, and oils for biofuels. These compounds may come from macroalgae and microalgae of various divisions including Chlorphyta, Rhodophyta, and Phaeophyta. Though beyond the scope of this review, prokaryotic Cyanobacteria also produce the already listed valuable compounds in addition to some others including neurotoxins.
Further examination of already studied marine algal species and their relatives is necessary for marine algae to truly become one of the great, well-known marine resources. Luckily, they are abundant and offer very little chance for over-exploitation. Their potential for production in an aquaculture setting is also a huge benefit in addition to their valuable secondary actions and products including carbon dioxide fixation and nitrogen waste production for fertilizer. When production becomes more economically feasible and more efficient, marine algae may represent the biggest breakthrough in marine natural product development for medicine and other products.
References
1. Bhadury, Punyasloke, and Phillip C Wright. “Exploitation of Marine Algae: Biogenic Compounds for Potential Antifouling Applications.” Planta 219.4 (2004): 561–78.
2. Vaughan, V C, M-R Hassing, and P a Lewandowski. “Marine Polyunsaturated Fatty Acids and Cancer Therapy.” British Journal of Cancer 108.3 (2013): 486–92.
3. Lloyd-Still, John D et al. “Bioavailability and Safety of a High Dose of Docosahexaenoic Acid Triacylglycerol of Algal Origin in Cystic Fibrosis Patients: A Randomized, Controlled Study.” Nutrition (Burbank, Los Angeles County, Calif.) 22.1 (2006): 36–46.
4. Ben-Amotz, A. “Production of-carotene from Dunaliella.” Chemicals from microalgae (1999): 196-204.
5. Krinsky, Norman I., and Elizabeth J. Johnson. “Carotenoid actions and their relation to health and disease.” Molecular aspects of medicine 26.6 (2005): 459-516.
6. Borowitzka, Michael A. “Microalgae as Sources of Pharmaceuticals and Other Biologically Active Compounds.” Journal of Applied Phycology 7 (1994): 3–15.
7. Del Campo, José a, Mercedes García-González, and Miguel G Guerrero. “Outdoor Cultivation of Microalgae for Carotenoid Production: Current State and Perspectives.” Applied microbiology and biotechnology 74.6 (2007): 1163–74.
8. Brennan, Liam, and Philip Owende. “Biofuels from microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products.” Renewable and Sustainable Energy Reviews 14.2 (2010): 557–577.
9. Black, Paleah L., John T. Arnason, and Alain Cuerrier. “Medicinal Plants Used by the Inuit of Qikiqtaaluk (Baffin Island, Nunavut)This Paper Was Submitted for the Special Issue on Ethnobotany, Inspired by the Ethnobotany Symposium Organized by Alain Cuerrier, Montréal Botanical Garden, and Held in Montréal at .” Botany 86.2 (2008): 157–163.
10. Dias, Daniel a., Sylvia Urban, and Ute Roessner. “A Historical Overview of Natural Products in Drug Discovery.” Metabolites 2.4 (2012): 303–336.
11. Arterburn, Linda M et al. “Algal-Oil Capsules and Cooked Salmon: Nutritionally Equivalent Sources of Docosahexaenoic Acid.” Journal of the American Dietetic Association 108.7 (2008): 1204–9.
12. Tapiero, H et al. “Polyunsaturated Fatty Acids (PUFA) and Eicosanoids in Human Health and Pathologies.” Biomedicine & pharmacotherapy = Biomédecine & pharmacothérapie 56.5 (2002): 215–22.
13. Baracos, Vickie E., Vera C. Mazurak, and David WL Ma. “n-3 Polyunsaturated fatty acids throughout the cancer trajectory: influence on disease incidence, progression, response to therapy and cancer-associated cachexia.” Nutrition research reviews 17.02 (2004): 177-192.
14. Gerber, Mariette. “Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies.” British Journal of Nutrition 107.S2 (2012): S228-S239.
15. Weed, Harrison G., et al. “Lean body mass gain in patients with head and neck squamous cell cancer treated perioperatively with a protein‐and energy‐dense nutritional supplement containing eicosapentaenoic acid.” Head & neck 33.7 (2011): 1027-1033.
16. Freedman, Steven D., et al. “Association of cystic fibrosis with abnormalities in fatty acid metabolism.” New England Journal of Medicine 350.6 (2004): 560-569.
17. Ward, Owen P, and Ajay Singh. “Omega-3/6 Fatty Acids: Alternative Sources of Production.” Process Biochemistry 40 (2005): 3627–3652.
18. Apt, Kirk E., and Paul W. Behrens. “Commercial developments in microalgal biotechnology.” Journal of Phycology 35.2 (1999): 215-226.
19. Ballantine, David L. et al. “Antibiotic Activity of Lipid-Soluble Extracts from Caribbean Marine Algae.” Hydrobiologia 151-152.1 (1987): 463–469.
20. Falch, B. S., et al. “Antibacterial and cytotoxic compounds from the blue-green alga Fischerella ambigua.” Planta Medica 58 (1992).
21. Kubanek, Julia, et al. “Seaweed resistance to microbial attack: a targeted chemical defense against marine fungi.” Proceedings of the National Academy of Sciences 100.12 (2003): 6916-6921.
22. Ben-Amotz, Ami, and Mordhay Avron. “The biotechnology of cultivating the halotolerant alga Dunaliella.” Trends in Biotechnology 8 (1990): 121-126.
23. Guerin, Martin, Mark E. Huntley, and Miguel Olaizola. “Haematococcus astaxanthin: applications for human health and nutrition.”TRENDS in Biotechnology 21.5 (2003): 210-216.
24. McKendry, Peter. “Energy production from biomass (part 1): overview of biomass.” Bioresource technology 83.1 (2002): 37-46.