Climate variability and life history impact stress, thyroid, and immune markers in California sea lions (Zalophus californianus) during El Niño conditions
By Isabelle Geller, SRC intern
There are many situations which may increase the level of stress in an animal – for example, not being able to eat enough food to meet energy demands or being in temperatures above or below a tolerable range they can. DeRango et al. (2019) aimed to study both of these factors with respect stress levels of California sea lions (CSL) (Figure 1). Specifically, the authors looked at impacts of environmental change (e.g. variability over the course of the study) and life history (e.g. pre-breeding and post-breeding stages – juvenile and adult – with different energy demands) on CSL during the El Niño of 2015-2016.

Figure 1: California Sea Lion (Rhododendrites [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]).
To conduct the study, blood samples were taken from juvenile and adult CSL during October 2015/ October 2016 and March 2016/ August 2016 respectively (Figure 2). From the blood samples they analyzed stress hormones, glucose levels, thyroid hormones, immune markers and interactions between the HPA (hypothalamus, pituitary gland, and adrenals glands) axis; which they hypothesized would all be suppressed. However, the results were a bit different from expected:
- From 2015 to 2016 the glucose and stress hormone levels for juvenile and adult male CSL decreased.
- From 2015 to 2016 the thyroid hormones for juvenile and adult male CSL increased
- From 2015 to 2016 immunoglobin increased in juveniles but decreased for adult male CSL after breeding.
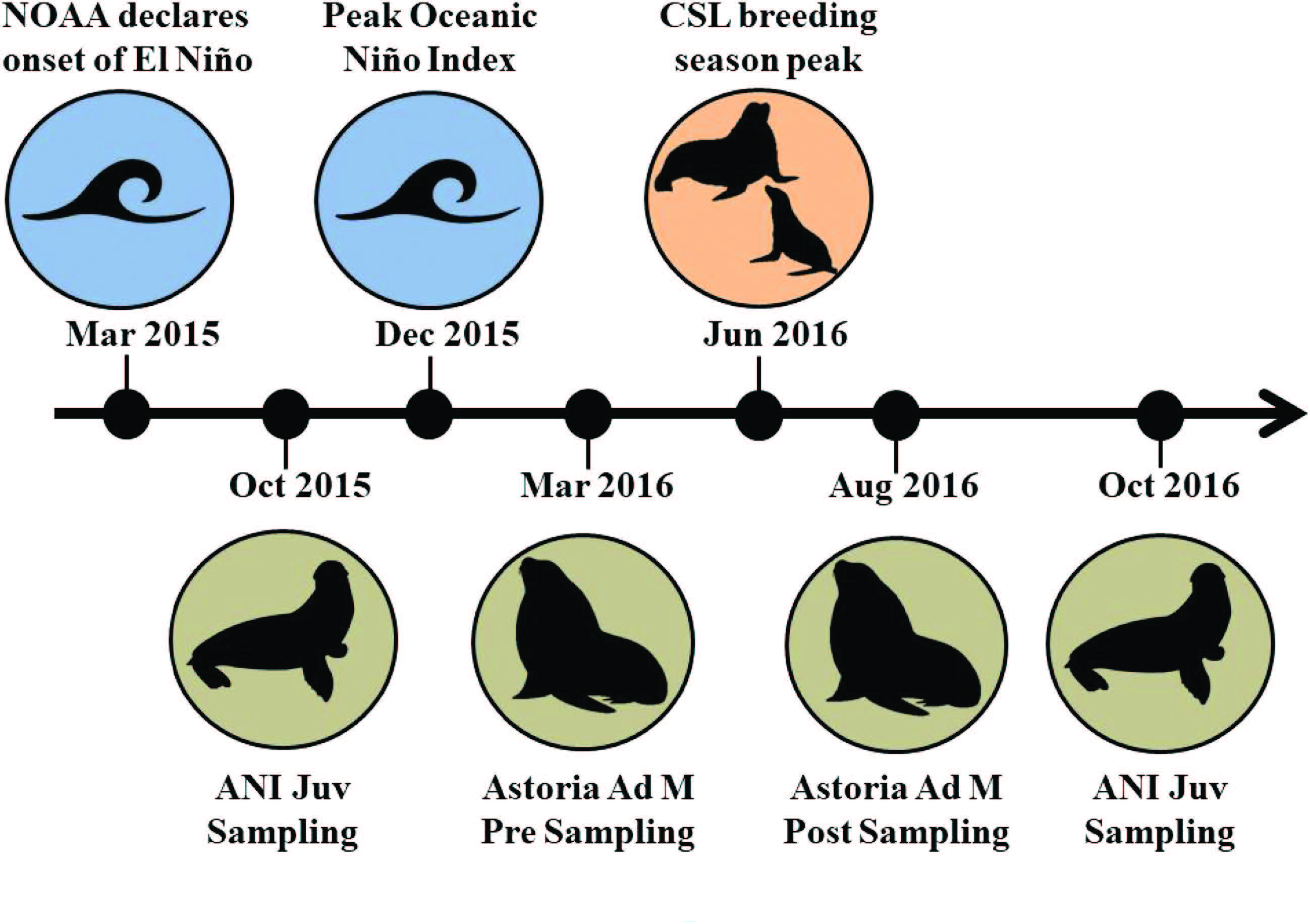
Figure 2: Timeline of events for the research of CSL. Types of CSL sampled: JUV = Juvenile, Ad M = Adult Male. Locations where CSL were sampled: ANI = Año Nuevo Island, California and Astoria, Oregon. (DeRango et al. 2019)
So, what do the results mean? The authors interpreted these trends as follows:
- From 2015 to 2016 the glucose and stress hormone levels for juvenile and adult male CSL decreased:
Since the juvenile sea lions had been facing chronic stress due to lack of food and the adult male sea lion due to sustained breeding period, they animals may have been unable to mount a normal stress response to the handling and drawing of blood. Additionally, due to a lack of nutrients for juvenile seals and the extremely energetically expensive reproductive process glucose (an important energy in organisms) decreased.
2. From 2015 to 2016 the thyroid hormones for juvenile and adult male CSL increased
To support energy intensive activities like hunting and breeding, thyroid function to increase during stressful activities like breeding and foraging. This may occur since the CLS were fasting, for aforementioned reasons, thus the stress hormone may not have had the same impact of suppression on the thyroid hormone as usual.
3. From 2015 to 2016 immunoglobin increased in juveniles but decreased for adult male CSL after breeding.
Immunoglobin, which is a markers for immune system cells, increased in juveniles likely due to greater exposure to pathogens, which would increase during El Niño events. However, immunoglobin decreased for adult male CSL, because reproduction and energetic limitations caused immunosuppression.
Looking to the future, this study has shown the impact of climate change on life history events and on the CSL population – this has contributed to an understanding of the marine mammal stress response to capture, and could help to create better research protocol for the CSL in the future.
Works cited
DeRango EJ, Prager KC, Greig DJ, et al (2019) Climate variability and life history impact stress, thyroid, and immune markers in California sea lions (Zalophus californianus) during El Niño conditions. Conserv Physiol 7:1–15. doi: 10.1093/conphys/coz010