By Casey Dresbach, SRC Intern
The pH of the ocean is changing incrementally, as a result of increases in atmospheric carbon dioxide. As shown in Figure 1, a proceeding decline in seawater pH has been induced by ocean acidification and will continue over the next hundred years. The ocean is expected to decline an average of 0.3 pH units by the end of the century. Changing the geochemistry of the ocean is consequently changing the biogeochemical processes of the sea, including marine microbial populations. The microbial community is extremely important to the marine carbon cycle in their role to decompose and recycle nutrients, as shown in Figure 2.

Figure 1. Predicted Ocean Surface pH Through the Year 2100.
The ocean maintains a viable level of carbon dioxide from such decomposition and subsequent respiration of organic molecules by heterotrophic bacteria. Since the industrial boom, the human obsession to industrialize is continually offsetting the balance of the ocean’s carbon dioxide levels and setting up a future of mutational distress. The burning of fossil fuels (primarily coal) and its emissions are manipulating the ocean for the worse.
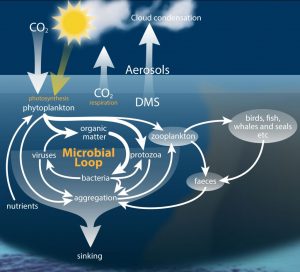
Figure 2. Microbial communities graze, break down, recycle and respire nutrients in the ocean helping to maintain an interconnected web of carbon transfer.
Researchers Ian Joint, Scott C. Doney, and David M Karl wrote an article for the ISME Journal regarding their perspectives on the question, “Will ocean acidification affect marine microbes?”
The reasoning behind their perspective has much to do with the lack of proper research done addressing the issue of microbial function in a lowered pH oceanic environment. Much is known about calcifying organisms, such as corals and coccolithiphores, in regards to ocean acidification. With an increase in acidity, maintaining calcite and aragonite shells and skeletons becomes nearly impossible.
Most studies that are published include discussions of the ocean pH in the context of geological time scales in spatial uniformity of pH for the present-day ocean. However, pH of the oceans is not constant and there are considerable seasonal, depth, and regional variations that come into play. With that in mind, pH is naturally variable and marine organisms-particularly microbes-must already be capable of adapting to rapid and sometimes large changes in pH. Kai T. Lohbeck, Ulf Riebesell and Thortsten B. H. Reusch published an article for Nature Geoscience regarding the topic of adaptive evolution of a key phytoplankton species to ocean acidification. According to the article, they suggested that contemporary evolution could help to maintain the functionality of microbial processes at the base of marine food webs in the face of global change; that these organisms are currently facing evolutionary adaptations.
The pH levels in the ocean are not consistent, especially when considering other variables such as light, temperature, and depth. The multifactorial relationship among these variables is direct. Researchers Ian Joint, Scott C. Doney, and David M Karl presented a null hypothesis to be tested: marine microbes posses the flexibility to accommodate pH change and there will be no catastrophic changes in marine biochemical processes that are driven by phytoplankton, bacteria, and archae.
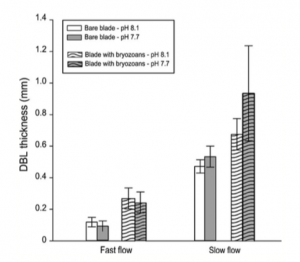
In an alternate scientific publication by J. Piontek, M. Lunau, N. Händel, C. Borchard, M. Wurst, and A. Engel, a study was conducted in regards to acidification increasing microbial polysaccharide degradation in the ocean. The ocean’s capacity for carbon dioxide storage is strongly affected by biological processes whose feedback potential is unfortunately difficult to evaluate. This article coincides with the previous considering that very little is known about potential effects ocean acidification poses not only on the microbial community themselves, but on bacterial degradation activity as a whole. Polysaccharides are a major component of organic matter, an energy source for the majority of bacterial communities. Their breakdown by bacterial enzymes seemed to have significantly accelerated during J. Piontek, M. Lunau, N. Händel, C. Borchard, M. Wurst, and A. Engel’s experimental simulation of ocean acidification. An experiment was set up to test the rate of enzymatic polysaccharide hydrolysis (how quickly bacteria could break down the macromolecule) in natural bacterioplankton communities. In this experiment, two environments were simulated: one being present day pH levels – what the ocean’s pH level is today and the latter being future day pH levels – what the ocean’s pH levels are predicted to be in the next hundred years. After each sample was incubated, concentrations of dissolved and particulate combined glucose, galactose, arabinose, and other sugars and acids were also detected. These concentrations represented the complexity of the relationships between the variables that come into play with acidification. This allowed the scientists to relate the differences in glucosidase activity (enzymes which break down glucose) and macromolecule concentration between present-day and future-ocean treatment to the increase in hydrogen ion concentration. Figure 3 illustrates the results of the above experiment with regards to an additional variable, permanent dark incubation and dark cycling incubation. The experiment revealed that the observed increase in glucosidase activity was directly proportional to the increasing acidity of the ocean water in a simulated acidification model. They also came to realize that changes in the enzymes’ activities reflected a community response of bacterioplankton to the simulated acidification as well. A collective conclusion of the experiment goes as follows: experimental results suggest that increased glucosidase activity at lowered seawater pH does not depend on the abundance of some specific bacterial strains, but reflects a community response to lowered seawater pH. This response could indicate that bacterial communities are currently undergoing adaptations to adjust to the decreasing pH. Higher enzymatic rates at lowered seawater pH are a chemical acidification effect on natural enzyme assemblages that is beneficial for the bacterial metabolism. Some might go on to further assume that come a more acidic oceanic environment, bacterioplankton communities will readily adapt and will not counter enhanced polysaccharide degradation. With a quicker degradation of polysaccharides, the bacterioplanktonic communities might flourish, quicker breakdown of sugars to be used as an energy source while simultaneously recycling nutrients back into the food web during degradation.

Figure 3. Percent loss of different macromolecules in present day oceanic pH levels and future pH levels with regards to another variable: permanent dark incubation and dark cycling incubation.
Results from different studies and perspectives show that a lot of variables must be considered when focusing on the question, “Will ocean acidification affect marine microbes?” Current analysis and comparison of freshwater pH and seawater pH is only so significant because the pH in freshwater acts on a short-term scale and the ocean acts on a far longer term. The ocean is too complex; there are too many variables to continue with the current broad-scale state of research.. Studies should go into the “micro-economics” of the ocean and its harmful relationship with acidification. There is more potential for a better understanding of a correlation between microbial communities and ocean acidification if things are broken apart and analyzed. Studying and analyzing the consequences of changes in individual variables, such as a potential threat to a major energy source of microbial communities (polysaccharides), can infer if there is in fact a present evolutionary adaptation of microbes to the increasingly acidic ocean. It’s also important to consider the possibility that microbial communities will adapt to the changes in pH but only to a certain degree before the rate of adaptation plateaus and these communities can no longer keep up with the consistent decrease in pH in the next one hundred years.
Resources
Ian Joint, S. C. (2010, June 10). Will ocean acidification affect marine microbes?. ISME Journal.
Piontek, M. L. (2010, May 19). Acidification increases microbial polysaccharide degradation in the ocean. Biogeosciences.
Kai T. Lohbeck, U. R. (2012, April 8). Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience.
Oakes, M. (2013, June). Changes: Forecase for Marine Mircrobial Communities. Retrieved February 21, 2016, from Antarctica: http://www.antarctica.gov.au/magazine/2011-2015/issue-24-june-2013/science/changes-forecast-for-marine-microbial-communities
Stocker, T. D. K. Climate Change 2013: The Physical Science Basis. Cambridge and NY,UK and USA: IPCC.