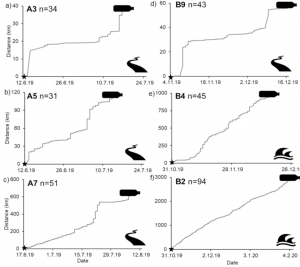
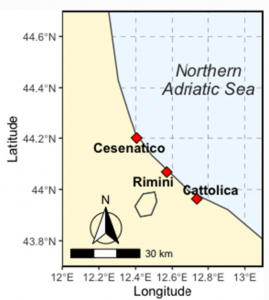
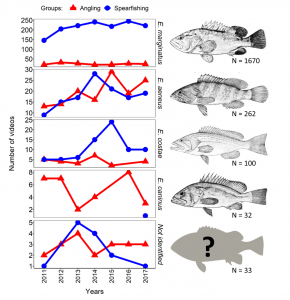
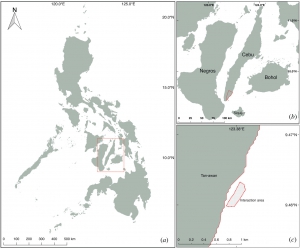
The Microplastic Problem
By Megan Buras, SRC intern

Figure 1: Plastic products are ubiquitous in our lives and the environment (Photo via Tanvi Sharma on Unsplash)
Today plastic is everywhere, from grocery stores to health products and even the shoes on our feet. The massive amount of plastic used daily, and its improper disposal have led to the accumulation of these plastics in the environment. Once plastic debris enters the ocean, it “breaks down into microplastics by photolytic, mechanical and biological degradation” (Alfaro-Núñez et al. 2021). These microplastics cause a wide range of issues, including ingestion by filter feeders mistaking this debris for plankton. One study reported “that 26%–52% of the fish collected in the English Channel had plastic debris in their gut” (Silva-Cavalcanti et al. 2017). The accumulation of microplastics in the environment has reached a point where it has begun to impact the species that live there. A study published in 2021 focused on the Tropical Eastern Pacific and Galápagos archipelago collected 240 samples from 16 different marine species. They found microplastic particles in 100% of the samples (Alfaro-Núñez et al. 2021). Species are now commonly encountering and ingesting microplastics. So what does this mean, and why is this an issue? In terms of the species that are primarily ingesting these microplastics, it has been found to cause intestinal issues, reduce nutrient absorption, and even impact fish metabolism and interfere with their immune system’s responses (Lu et al. 2016, Silva-Cavalcanti et al. 2017). Fish species that humans commonly consume have been found to contain microplastics, but little research has been done on how microplastic consumption affects humans.
Fish and aquatic products make up a significant portion of the diet of many people globally. The amount of global aquatic animal food production has “increased over threefold from 40.8 million tonnes in 1970 to 128 million tonnes in 2010”(Tacon and Metian 2013). It is evident that fisheries play an important role in global food security. This demand for increased aquatic food production has led to damaging and catastrophic effects on the global fish stocks, threatening the food security of the world while trying to meet its demands. The rise of aquaculture has also been significant but comes with its own series of challenges in terms of what is used to feed the fish as well as disease management and resource usage.

Figure 2: Commercial fishing has been found to have an important connection to microplastic pollution (Photo via Megane Delhaie on Unsplash)
On top of all that information, a study has found that commercial fishing contributes significantly to microplastic pollution. Results from the study “showed that the dominant contaminants (polypropylene fibers and polyethylene fibers) might originate from the abrasion of fishing gear and contributed to 61.6% of the total abundance of microplastics in surface sediment”(Xue et al. 2020). Overfishing and microplastic pollution are connected in more ways than previously thought. This begs the question: How can we better manage global fisheries productively and efficiently without compromising microplastic pollution?

Figure 3: Global food security depends on the stability of fish stocks and proper management (Photo via David Todd McCarty on Unsplash)
Looking to the future, what can be done to reduce microplastic accumulation in the environment? Reducing the demand for plastic-packaged products and plastic microbeads could potentially cut off the source of plastic debris. Better regulation and disposal of plastic could limit the amount entering the ocean and environment. What can be done to protect fisheries? More effective management of commercial marine fisheries to sustain the global fish stocks for future generations. And, of course, more research into the effects of microplastic ingestion in humans would be an excellent place to start too.
Works cited
Alfaro-Núñez, A., D. Astorga, L. Cáceres-Farías, L. Bastidas, C. S. Villegas, K. Macay, and J. H. Christensen. 2021. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Scientific reports 11:1-8.
Lu, Y., Y. Zhang, Y. Deng, W. Jiang, Y. Zhao, J. Geng, L. Ding, and H. Ren. 2016. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environmental science & technology 50:4054-4060.
Silva-Cavalcanti, J. S., J. D. B. Silva, E. J. de França, M. C. B. de Araújo, and F. Gusmão. 2017. Microplastics ingestion by a common tropical freshwater fishing resource. Environmental pollution 221:218-226.
Tacon, A. G., and M. Metian. 2013. Fish matters: importance of aquatic foods in human nutrition and global food supply. Reviews in fisheries Science 21:22-38.
Xue, B., L. Zhang, R. Li, Y. Wang, J. Guo, K. Yu, and S. Wang. 2020. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment. Environmental science & technology 54:2210-2217.