Harmful Algal Blooms and Climate Change: Exploring Future Distribution Changes
By: Chris Schenker, SRC Intern
Across the globe, the effects of climate change are manifesting. Due to anthropogenically-induced environmental changes, the geographic occurrence of many species is being altered, and algae is no exception. Under the right environmental conditions, some algal species can cause harmful algal blooms (HABs) which create toxins and produce many harmful side effects. Fisheries are affected by HABs, as are some filter feeding species. Many commercially important bivalves, such as shellfish, retain these harmful chemicals in their tissue for up to six months. This means that one prolonged algal bloom can close coastal aquaculture and fisheries for months at a time, creating negative economic impacts and posing a threat to public health. Vulnerable species and ecosystems are also sensitive to HABs, and one bad event can push a species to extinction or make long lasting ecosystem-wide changes.

Figure 1: Many commercially important species of shellfish are under threat due to harmful algal blooms. (Source: Unsplash.com)
Worldwide, blooms have increased in frequency and impact. Different algal species are affected in different ways by environmental conditions, but it is possible that manmade changes have played a part in this. Many HABs that used to only occur at lower latitudes have crept north in recent years, and in this paper, Townhill et al. (2018) aimed to use species distribution modeling to provide a broad overview for “changing geographic affinity” (p. 1884). Using a high-resolution climate model integrated into global climate model outputs, the authors were able to incorporate a species’ global environmental exposure into its habitat suitability function. The “habitat suitability function” was constructed from data on species occurrence for a number of algal species, with an emphasis placed on variables most believed to affect algal occurrence, such as near bottom and sea surface temperature and salinity, differences between the surface and bottom values for each, and bathymetry. Next, a “relative habitat suitability” score between 0 and 1 was generated by running the data through the Maximum Entropy (Maxent) model which describes hydrographic and bathymetry conditions that a species currently seems to favor. Finally, the model was used to generate predictions of the latitudinal center of a species’ distribution in the near-term (2040-2069) and long-term (2069-2098). The estimates were used to understand how a species general distribution may change from the present.
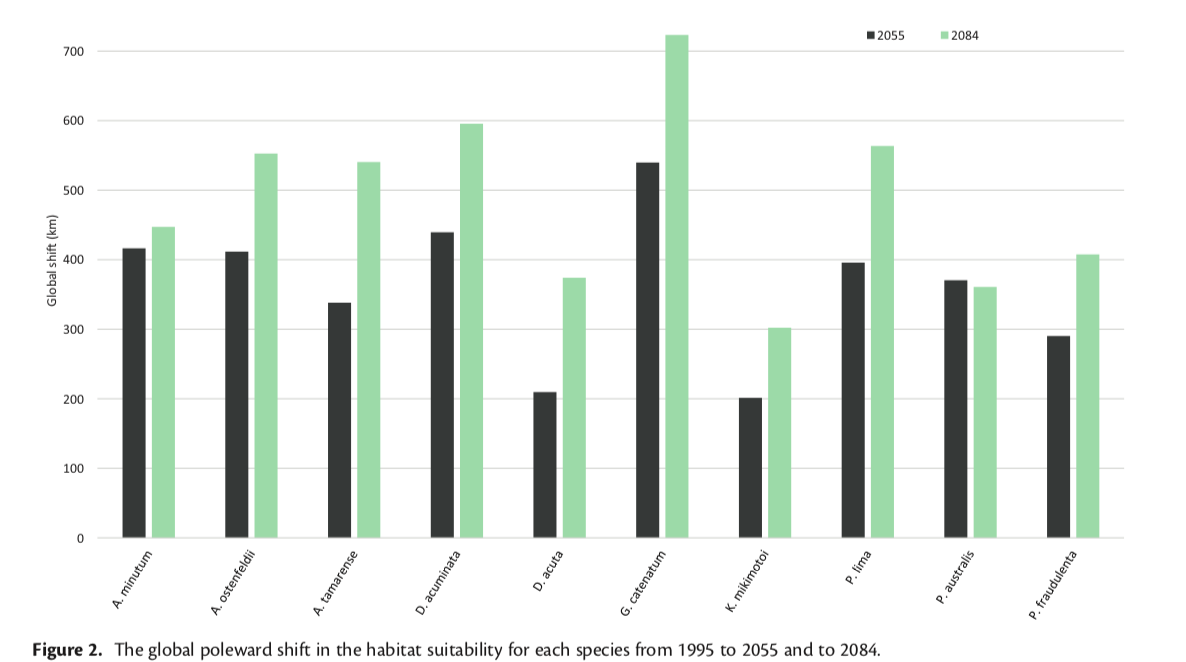
Figure 2: All species studied are expected to experience a global shift towards the poles. (Townhill et al., 2018)
Every species studied was projected to experience a northward global shift, with most of the change occurring at end of the century. Bathymetry and near bed temperature were found to be the variables with the greatest contribution to model fit. All but three species experienced a northward shift in the Northern European shelf seas, with D. acuta and Gymnodinium catenatum having the greatest at 800-1000 km northwards for mid and end of century. G. catenatum was also predicted to have the largest northwards shift globally with an estimate of more than 700 km.
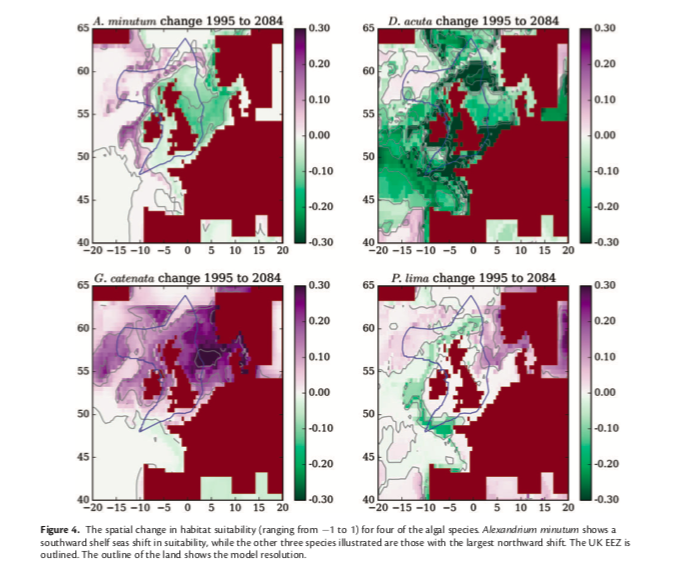
Figure 3: Alexandrium minutum is predicted to have a southward shift in shelf seas, while the other three species shown are expected to have the greatest northward shift. (Townhill et al., 2018)
Although there are differences in species-specific predictions compared to other studies, the work of Townhill et al. (2018) highlights the need for near- and long-term forecasting to understand the risk of future algal species redistribution. More sophisticated future models will likely lead to better predictions, allowing researchers to stay abreast of ecological trends. However, it is also important to understand that relative suitability helps us understand which species might become more prevalent and therefore need closer monitoring, but it is impossible to predict blooms based on abundance data alone. For that, local and near-term environmental conditions are much more important. Thus, this brand of species distribution modeling is meant to supplement conventional monitoring efforts, not replace them.
Work Cited:
Townhill, B. L., Tinker, J., Jones, M., Pitois, S., Creach, V., Simpson, S. D., Dye, S., Bear, E., and Pinnegar, J. K. Harmful algal blooms and climate change: exploring future distribution changes. ICES Journal of Marine Science, 75: 18821893.