What are harmful algal blooms (HABs) and why do they affect so many marine organisms?
By: Brenna Bales, SRC Intern
One cannot live in the state of Florida and be unaware of the effects of a red tide. It’s lethal destruction spurs concern from conservationists, divers, and fishermen alike. What begins as plankton at the basis of the food web, supplying nutrients to shellfish and larvae, gets sent into overdrive and wreaks havoc on marine and brackish ecosystems (Halegraeff 1993). How can such small organisms be responsible for so much harm?
Natural biogeochemical cycles, such as nutrient upwelling and salinity cycles, are responsible for creating plankton blooms composed of dinoflagellates, diatoms, or cyanobacteria (Sellner et al. 2003). A lethal domino effect is created when their populations explode. When zooplankton respire, they require oxygen and nutrients. In a bloom situation, the large amount of plankton may use up so much oxygen in the water that there is hardly any left for other animals that need it such as fish, crustaceans, and shellfish. These animals can then suffocate and die underwater (Anderson 2009). There are many occurrences of natural plankton blooms that we can observe, but an increasing number are attributable to anthropogenic effects (Anderson et al. 2008, Sellner et al. 2003). Humans, by fertilizing golf courses, personal lawns, and sewage dumping, can release organic nutrients into waterways which supply these plankton with nourishment, exploding their numbers.
A red tide is only one example of what is termed a harmful algal bloom (HAB). Over 300 species of plankton can contribute to a red tide, but there are other HABs that can be fatal to humans and animals such as paralytic shellfish poisoning, diarrhetic shellfish poisoning, amnesic shellfish poisoning, and ciguatera (Halegraeff 1993). Ciguatera is the most serious, affecting more than 50,000 humans annually (Anderson 2012). The mechanism by which these cause harm to the marine environment is not by oxygen depletion, but rather toxin accumulation. More and more toxins are discovered every year as research continues, which includes classes such as brevetoxins, saxitoxins, and anatoxins (Halegraeff 1993). These toxins accumulate in plants or small crustaceans/shellfish, which fish will eat. Larger predatory animals such as dolphins or sharks will then consume the infected fish and become poisoned. This is also known as bioaccumulation and can occur with any harmful substance making its way up the food chain such as mercury. When the algal blooms occupy many square miles, these animals can die in mass numbers and wash up on shore or float to the surface in large aggregations (Figure 1).

Figure 1. A red tide event causes fish to wash up on a popular beach near Tampa Bay, Florida. (http://wusfnews.wusf.usf.edu/post/pinellas-cleaning-beaches-red-tide-arrives)
There are three mechanisms by which a marine animal may become detrimentally affected by a HAB. The first is by ingestion, as described above. The second is by inhalation. This mainly affects marine mammals that come to the surface to breath air (Alcock 2007). Toxins from HABs can become aerosolized when algae burst underwater and release these toxins into bubbles that float up to the surface and explode into the air (Pierce 1986). The proximity of these airborne molecules to the water makes it easy for the particles to become inhaled by animals breathing at the surface such as whales and dolphins, thus infecting their airways. The third mechanism is by touch. Brevetoxins have been shown to be able to absorb into skin cells, and when animals’ skin in the water comes into proximity with a HAB, they can become infected (Kemppainen et al. 1991). For marine mammals and humans, all three mechanisms can combine ensuring a deadly fate.
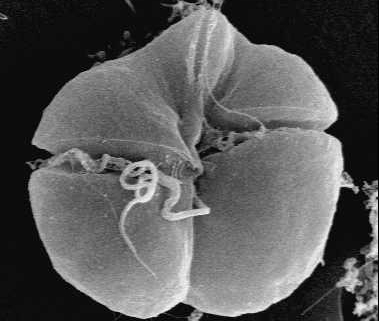
Here in Florida there a well-known, HAB-causing organism: the dinoflagellate Karenia brevis (Figure 2). It is the most significant HAB-causing organism in the Gulf of Mexico (Anderson et al. 2008). K. brevis is a brevetoxin, which produces neuro and muscular toxins (Kirkpatrick et al. 2004). When infected fish are consumed by humans, they can cause nausea, vomiting, paresthesia (burning/tickling sensations) in the mouth, lips, and tongue, dizziness, slurred speech, and partial paralysis (Watkins et al. 2008). A recent study has shown that K. brevis can even affect the economically-important scleractinian coral species in Florida, by upsetting protein expression, metabolism, and oxygen production (Reynolds 2018).
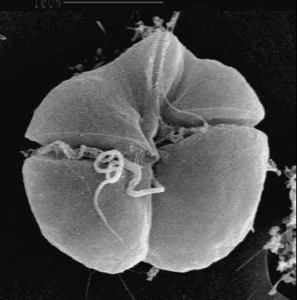
Figure 2. Dinoflagellate Karenia brevis. Also known as Gymnodinium breve (Davis 1948), Ptychodiscus brevis (Steidinger 1979) (Wikimedia Commons).
Florida is flanked by oceans on both coasts, creating water-based industries such as snorkeling/diving, fishing, and going to the beach. Many people live on waterways that are susceptible to HABs, rendering them vulnerable to airborne effects. Combined impacts on public health, commercial fishing, recreation and tourism, and management cost the state an average $450 million annually from 1987-1992 (Anderson et al. 2000). Prevention and mitigation are essential for alleviating some of these negative impacts, especially if more and more HABs are being observed globally each year (Anderson 2012). As human populations become increasingly coastal, we must work together on finding solutions to this growing problem for both the sake of our oceans, and for ourselves.
Works Cited:
Alcock, F., 2007. An assessment of Florida red tide: causes, consequences and management strategies. Marine Policy Institute, Mote Marine Laboratory, Sarasota, FL.
Anderson, D.M., Hoagland, P., Kaoru, Y. and White, A.W., 2000. Estimated annual economic impacts from harmful algal blooms (HABs) in the United States (No. WHOI-2000-11). NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION NORMAN OK NATIONAL SEVERE STORMS LAB.
Anderson, D.M., Burkholder, J.M., Cochlan, W.P., Glibert, P.M., Gobler, C.J., Heil, C.A., Kudela, R.M., Parsons, M.L., Rensel, J.J., Townsend, D.W. and Trainer, V.L., 2008. Harmful algal blooms and eutrophication: examining linkages from selected coastal regions of the United States. Harmful Algae, 8(1), pp.39-53.
Anderson, D.M., 2009. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean & coastal management, 52(7), pp.342-347.
Anderson, D., 2014. HABs in a changing world: a perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change. In Harmful algae 2012: proceedings of the 15th International Conference on Harmful Algae: October 29-November 2, 2012, CECO, Changwon, Gyeongnam, Korea/editors, Hak Gyoon Kim, Beatriz Reguera, Gustaaf M. Hallegraeff, Chang Kyu Lee, M. (Vol. 2012, p. 3). NIH Public Access.
Hallegraeff, G.M., 1993. A review of harmful algal blooms and their apparent global increase. Phycologia, 32(2), pp.79-99.
Kemppainen, B.W., Reifenrath, W.G., Stafford, R.G. and Mehta, M., 1991. Methods for in vitro skin absorption studies of a lipophilic toxin produced by red tide. Toxicology, 66(1), pp.1-17.
Kirkpatrick, B., Fleming, L.E., Squicciarini, D., Backer, L.C., Clark, R., Abraham, W., Benson, J., Cheng, Y.S., Johnson, D., Pierce, R. and Zaias, J., 2004. Literature review of Florida red tide: implications for human health effects. Harmful algae, 3(2), pp.99-115.
Pierce, R.H., 1986. Red tide (Ptychodiscus brevis) toxin aerosols: a review. Toxicon, 24(10), pp.955-965.
Reynolds, D.A., 2018. The effects of the red tide producing dinoflagellate, Karenia brevis, and associated brevetoxins on viability and sublethal stress responses in scleractinian coral: a potential regional stressor to coral reefs.
Sellner, K.G., Doucette, G.J. and Kirkpatrick, G.J., 2003. Harmful algal blooms: causes, impacts and detection. Journal of Industrial Microbiology and Biotechnology, 30(7), pp.383-406.
Watkins, S.M., Reich, A., Fleming, L.E. and Hammond, R., 2008. Neurotoxic shellfish poisoning. Marine drugs, 6(3), pp.431-455.